Archive for December 2016
Call for submissions: Would you like to present at IKC-Regener8 Conference 2017?
Like last year, we’re opening up a session of the conference for crowdsourced content. This means anyone (who fulfils the criteria) can apply to present. The proposals judged the best (by the event committee) will go to a vote across the IKC-Regener8 and wider med tech community to decide who they most want to see […]
Read More
World-leading medical engineering shown to Minister
Cutting-edge developments in medical technology have been demonstrated to Government Health Minister Lord Prior, during a fact-finding mission to Leeds. Both the University of Leeds and Leeds Teaching Hospitals NHS Trust have built up significant expertise in this field, much of it through partnership working and sharing the expertise of staff. The University has a […]
Read More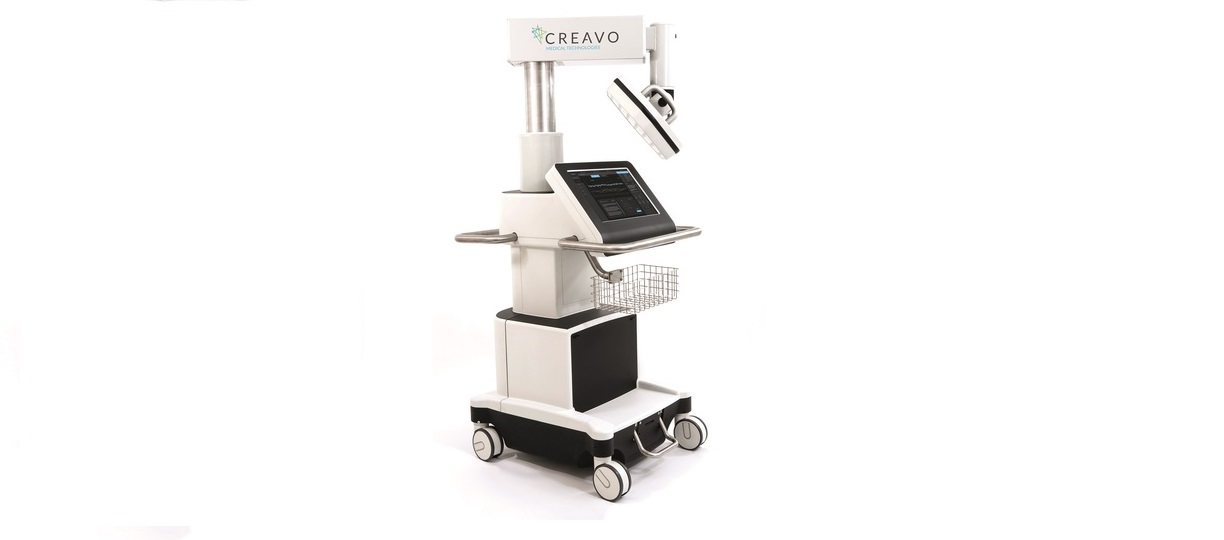
Creavo’s Vitalscan receives CE mark
Creavo’s Vitalscan receives CE mark Creavo Medical Technologies, an emerging British company developing diagnostics technology for acute medical settings, is today (24 November 2016) pleased to announce that its first device, Vitalscan, has received CE mark registration, enabling market launch in Europe. The successful receipt of the CE mark means that the device will now […]
Read More
University to lead medical technologies audit
A University of Leeds-led consortium has been chosen by the Government to carry out an audit of research, innovation and industry in medical technologies. The Science Innovation Audit contract, announced in today’s Autumn Statement, was awarded in recognition of the Leeds City Region consortium’s significant expertise and strength in this field. The audit will explore how […]
Read More
Toshiba Fellowship Programme 2017 – Applications Now Open
The Toshiba Fellowship Programme offers outstanding scientists an annual opportunity to apply to join Toshiba’s research and development laboratories in Japan for up to two years, on a Research Fellowship Contract. The Toshiba Fellowship Programme is a unique opportunity for recently qualified PhD level scientists, mainly from science, computing and mathematics disciplines. It offers a […]
Read MoreUniversity of Leeds awarded £3.7m to fund knee therapy research
The University of Leeds has been awarded £3.7m by the EPSRC to develop novel testing methods for new knee therapies to treat osteoarthritis and other conditions. The research at Leeds will be led by Professor Ruth Wilcox, Director of the Institute of Medical and Biological Engineering, and will aim to develop novel testing methods for new knee […]
Read MoreMed Tech Industry urged to bid in partnership for £100,000 proof-of-concept funding
Medical technology companies working in Regenerative Devices and Medical Devices with Enhanced Regenerative Functions are being urged to co-develop new translational research proposals with academia for a newly launched funding call by the Medical Technologies IKC. Grants of up to £100,000 are available for successful collaborative proposals. Following a successful portfolio of translational projects, the […]
Read More
New £816 million investment in health research
Leeds Musculoskeletal Biomedical Research Unit (LMBRU) has been awarded funding for the only dedicated musculoskeletal biomedical research centre in the UK. As part of a new package of research funding from the government, the LMBRU will receive nearly £7 million to continue world-leading research on musculoskeletal conditions. The funding has been awarded to 20 NHS […]
Read MoreApply for translational proof-of-concept funding: Regenerative Devices Industry Partnership Call
Academics and researchers working in close collaboration with medical technology industry partners can apply for up to £100,000 FEC* from the Medical Technologies IKC to undertake proof of concept projects in Regenerative Medical Devices & Medical Devices with Enhanced Regenerative Functions. Regenerative devices is a growing area that focuses on functionally repairing and regenerating the patient’s […]
Read MoreIs your App a medical device? It’s healthy to know: regulator issues updated guidance
Updated guidance has been issued to help identify the health apps which are medical devices and make sure they comply with regulations and are acceptably safe. The full press release can be read on the gov.uk website at https://www.gov.uk/government/news/is-your-app-a-medical-device-its-healthy-to-know-regulator-issues-updated-guidance
Read More
