News
Creavo’s Vitalscan receives CE mark
Date: November 25, 2016
Creavo’s Vitalscan receives CE mark
Creavo Medical Technologies, an emerging British company developing diagnostics technology for acute medical settings, is today (24 November 2016) pleased to announce that its first device, Vitalscan, has received CE mark registration, enabling market launch in Europe.
The successful receipt of the CE mark means that the device will now start a largescale, multi-centre clinical trial at four of the UK’s major A&E departments, namely Bristol, Sheffield, – Leicester and Nottingham, followed by a second stage starting at three centres in the United States at Mayo Clinic Rochester, Cincinnati and Baylor Texas.
Early indications from smaller sets of clinical trials indicate that Vitalscan could meet an urgent clinical need for improving the current triage process for patients entering emergency departments with chest pain. The device uses advanced quantum principles to measure, display and store electromagnetic fluctuations caused by heart activity through a simple, non-invasive three to five-minute scan at a patient’s bedside. This scan accurately and quickly rules out significant ischaemic heart disease, such as heart attacks. The device will enable clinical practitioners, who operate in acute medical settings, to ‘rule out’ heart-related problems and prevent healthy patients from having to go through the current lengthy, and costly, chest pain triage process, which includes a variety of diagnostic tests including multiple ECGs and cardiac biomarker blood tests.
Dr Richard Charles, Emeritus Consultant Cardiologist at Liverpool Heart and Chest Hospital and Chair of Creavo Medical Technologies’ Medical Advisory Board, said: “There is compelling early evidence that Magnetocardiography (MCG) as utilised in Vitalscan, can quickly and accurately rule out suspected heart attack in emergency chest pain patients, rapidly relieving both patient anxiety and health care costs. The development of Vitalscan opens an exciting new chapter in understanding and managing heart disease.”
Steve Parker, CEO of Creavo, said: “Receiving the CE mark for Vitalscan means that we have achieved one of our critical strategic ambitions for 2016.
“At a time when the NHS is facing unprecedented pressures, Vitalscan has the potential to radically disrupt the current process for patients entering emergency departments with chest pain, bringing millions of pounds of savings to the NHS a week, easing pressure on bed space in A&E and reducing patient anxiety. The CE mark means that we can now look forward to building momentum with the launch of our multi-centre clinical study, testing the device in four of the busiest A&E departments in the UK and in the US.”
For further information, please contact:
Annabel John, Speed Communications
07771 916881
Leander Clarke, Speed Communications
07985 238713
Notes to editors
About Creavo Medical Technologies Ltd
Creavo Medical Technologies Ltd is a UK-based, privately held medical device company that is developing innovative diagnostic techniques. The company was formed in 2014 to bring to market ground-breaking technology developed by Professor Ben Varcoe, Chair of Quantum Information Science at Leeds University. It has facilities in Leeds and Coventry, UK.
Focused on improving front line patient care in acute medical settings, Creavo’s first application of this technology is the Vitalscan – a device that meets an urgent clinical need in global cardiology to more accurately ‘rule out’ heart-related problems at point of admission to emergency departments.
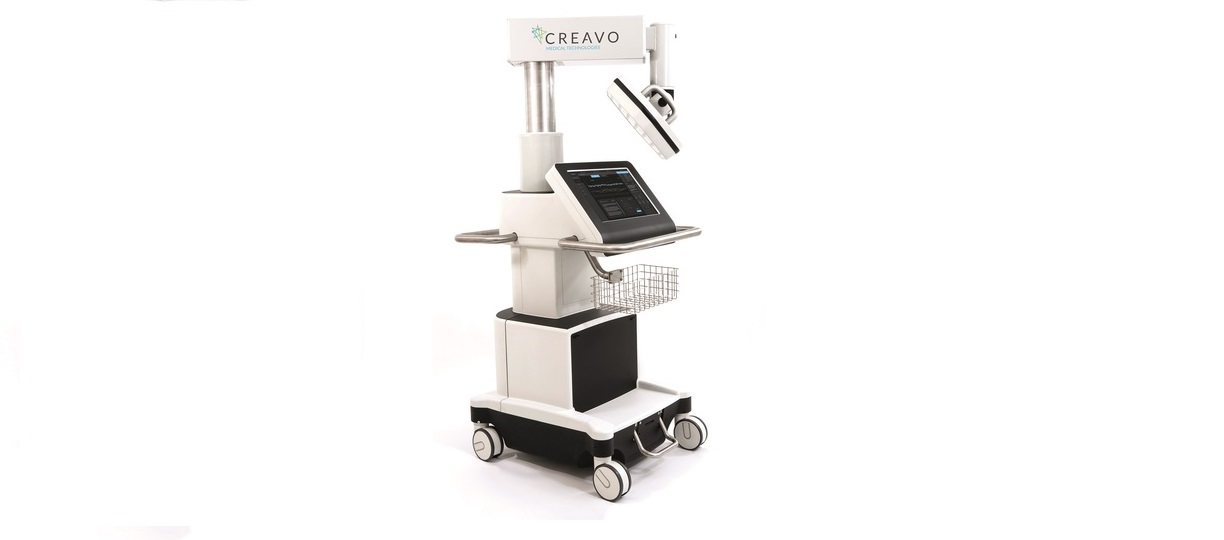
Vitalscan is a battery-powered, portable medical device that uses advanced quantum principles to measure, display and store electromagnetic fluctuations caused by heart activity. Early indications from smaller sets of clinical trials indicate that the device can accurately and quickly rule out significant ischaemic heart disease with near 100% accuracy through a simple, non-invasive three to five-minute scan at a patient’s bedside.
For more information about Creavo, please visit: http://www.creavomedtech.com/
Back to News Overview
